Make Sure your Browser has Java running and enabled .
If the simulation does not run click here: Java
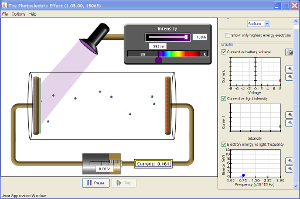
Objective use the same process described in class and determine the rules of photoelectric emmission
Identify related variables
Identify trends maxs and mins
A diagram should be in your notes as well
You may discuss on the Blog and work collaboratively
I already installed the newest version of Java, but when I click on the simulation, an error pops up saying that the file is either not supported or has been damaged. Does anyone have any suggestions?
ReplyDelete-Kevin Meglathery
use chrome and say "keep" when it asks
DeleteThey same thing happens. I click keep, try to open it, and it still does not work. Do I need to change any settings?
Delete-Kevin Meglathery
What browser are you using?
ReplyDeleteThis is what I found so far:
ReplyDeleteIntensity: number of electrons flowing (directly proportional)
Voltage: speed of electrons (directly proportional)
Current: Produced from the flowing of electrons, or intensity
Wave Length: speed of electrons (indirectly proportional); electrons flow when wave length is less than 540 nm (Sodium only - this changes according to the element)
If anyone agrees or disagrees, let me know.
The wavelength that the electrons flow changes depending on the voltage, but at 0 volts it agree its around the 540 nm mark.
Deletethe speed of the electrons is determined both by the energy (frequency/inverse of wavelength) AND the voltage
Also only for sodium i haven't observed the others.
I also found that when the energy of the electrons is very low (the photons have a high wavelength) then almost every one makes the jump, but when the energy is very high (small wavelength, like violet) then some electrons seem to not make the jump, this could just be a lag in my computer or something though
The electrons flow towards the "positive" side as well. For example, if you switch the orientation of your battery so that the + and the - are opposite, then the electrons won't make the jump, or will start the jump and be pulled back. So i feel like they travel in the opposite direction of the current? I feel like i'm missing something here.
Also, when the wavelength of the light gets longer (less frequency, less energy) a high amount of the electrons are considered to be of the "highest energy"
What i mean by this is when you are on violet light, and you check the box on the right that says "show only highest energy electrons", only very few appear. However, when you go to a high wavelength like 450, a higher number of "highest energy electrons" appear.
The electron flow does not rely on current, as they still flow when the current is 0
Max Current: 1.729 @ 196 nm, 100% intensity, 8 volts
Delete(all else equal 196 gave highest current)
Current is constant for all positive voltages, for negative voltages it slopes off to zero.
Intensity effects the maximum current
Sodium gives the max current and the widest range of useable wavelengths
I agree on all counts. By inference with your description (although I explicitly wrote it down) I said with 100% intensity current does not rive above 0 unless wavelength is under 540.
ReplyDeleteThat's when using sodium, correct?
DeleteYes
DeleteCan someone remind me of what we have to graph?
ReplyDeletei'd assume we have to graph max and min trends for each of the targets- just a guess
Deleteok nevermind, i take that back- maybe just by playing around with the current vs battery voltage and current vs light intensity?
DeleteI wasn't in class today could anyone tell me what I missed?
ReplyDeleteWhen using sodium as the target i found:
ReplyDeletefor all positive voltages (0.00-8.00) at 100% intensity the max current is 1.729 at 196 nm. and the minimum current is 0 which occurs at 491 nm and above.
However for the negative voltages the current is not constant like the positive voltages
For -8.00 V the maximum current is .463 at 100 nm. the minimum current is 0.00 at 122 nm and above
For - 6.00 V the maximum current is .882 at 100 nm the minimum current is 0.00 at 152 nm and above.
For -4.00 V the maximum current is 1.050 at 119 nm. the minimum current is 0.00 at 196 nm and above.
For - 2.00 V the maximum current is 1.315 at 149 nm. the minimum current is 0.00 at 290 nm and above
Intensity affects the amount of electrons (directly proportional)
ReplyDeleteThe lower the wavelength, the faster the electrons move (inversely proportional)
Negative voltage sends electrons back into the plate they left, the lower the voltage (-) the less far they go from their plate, lower wavelength (-) more electrons
Most electrons in between IR and UV (480NM).
Electrons only leave the side the light hits.
The variables are current, voltage, intensity, and frequency.
ReplyDeleteThe highest current for Sodium is 1.729 and this occurs only when it is at 196 nanometers in ultraviolet.
Intensity and current are directly related.
The higher the voltage, the higher attraction of the electrons.
Energy and frequency are directly proportional
At -4 volts with high intensity no electrons will reach other plates because there isn't enough kinetic energy.
Changing the metal affects the number of electron emissions.
Energy is only effected by frequency.
The only thing that changes when you change the metals is energy.
When wavelength is increased, energy is decreased.
If wavelength is greater than 494 nanometers, there is no energy.
Calcium has zero energy at 395 nm.
Platinum has zero energy at 188 nm.
Copper has zero energy at 251 nm.
Zinc has zero energy at 273 nm.
Some other stuff that is important to know when studying the photoelectric effect:
ReplyDeleteWhen light shines on a metal surface, it emits electrons
Experiment is done in a vacuum (cylindrical shape in the Java link)
Increasing intensity makes for increasing electrons
Increase in times makes for an increase in energy
The results for a metal will always be the same for that metal
Sodium: 197 nm wavelength produces the most current. Past 488 nm, no current is produced. When intensity is half, all values for current at a specific wavelength are also half. Positive voltage does not make a higher current, but negative voltage reduces the current. Voltage changes the speed of the particles while intensity changes the number of particles.
ReplyDeleteThings unmentioned (as far as I know) as of yet:
ReplyDeleteThe chance of the photon hitting the metal and having it give off an electron increases as one goes from the wavelength at which a photon can begin to make the metal give off electrons down (in wavelength).
The points at which there is no current produced due to the electrons never reaching the other side due to the electric field from the plates are
(I used the wavelength at which the I found the most current is produced due to the photoelectric effect):
Element / I=0A @ V=? / Wavelength Used
Sodium / -4.00V / 196 nm
Zinc / -3.98V / 149 nm
Copper / -4.24V / 138 nm
Platinum / -4.07V / 119 nm
Calcium / -3.39V / 179 nm
?????? / -4.01V / 160 nm
The initial velocity of the electron given off goes up as the wavelength goes down.
Mr. Craine,
ReplyDeleteI just got home and was hoping to experiment with this photo electric effect but the software cannot open on my computer. I know you put that link down to update or install your java but the virus I think my computer has keeps transferring me to these shady websites that want me to update everything but will definitely just destroy my computer. I will try to run over to my dads house tomorrow morning to use his computer which hopefully will work.
~Taylor Nardone
nevermind shes werkin now
DeleteWorking with sodium...
DeleteMaximum Current: 1.729
Intensity: 100%
Voltage: 8v
Wavelength: 196nm
Frequency 1.53E15 (honors chem math get at me)
Observations:
-Voltage and current seem to act in a direct relationship
-For the most part wavelength and current act in an inverse relationship, however 196nm is the point required for maximum current, as moving past it on either side decreases overall current
-Frequency and current act in the same manner, only their relationship is direct for the most part
-If the voltage is set below zero, the electrons seem to fail to make the jump, and then are attracted back against the flow of current
-intensity and current have a direct relationship
Sorry for the fact that a lot of this may have already been said but I haven't had time to read over the blog yet
~Taylor Nardone
Does anyone know if there is an alternate way to access this file
ReplyDeletephet.colorad.edu
ReplyDelete